As part of an ongoing series, Newry is interviewing experts and players across the carbon capture, utilization, and storage (CCUS) value chain to gain a perspective on where this exciting new industry is headed. To stay informed of future installments, click here.
Our interviewee has worked with carbon capture technologies since 2011. They are now employed as a materials scientist by Battelle at the National Energy Technology Laboratory (NETL) run by the Department of Energy (DOE) in Pittsburgh, Pennsylvania. At NETL, they study several carbon capture technologies—from sorbents, to solvents, to membranes—and next-generation materials. They also actively review external carbon capture research studies that have applied for DOE funding.
When you look at the DOE today, how much focus is there on carbon capture versus other decarbonization technologies like renewables or hydrogen?
The carbon capture program at the DOE is not new, it’s about two decades old. Over that time, there have been ups and downs in the funding levels, but the DOE has always wanted to maintain its carbon capture research. In the past couple of years, I would say the focus on carbon capture has been increasing drastically, both in terms of point-source capture and more recently direct air capture. So overall, I would say the DOE’s attention on CO2 capture has increased for sure.
It seems there’s an increasing focus not just on fundamental carbon capture research but now on pilot and demonstration projects—can you talk a little about that?
Yes, for instance, in the early 2000s and 2010s, basic science in the form of material discovery had more momentum. Nowadays, demonstration projects are getting more funding. There is still quite a bit of research going on for discovering new materials—new sorbents, for example—but the focus is now more on commercialization, moving technologies from bench scale to pilot and demonstration scale. As a result, more startups are getting funding. There’s still a considerable amount of money going to academic groups to discover or develop new materials, but the focus is more on demonstration projects according to the recent announcements.
The DOE now seems to have more money than ever to spend on carbon capture with the passage of the Bipartisan Infrastructure Law. Has the way in which the DOE deploys funding changed at all as a result?
According to the recent budget announcements on the DOE’s website, it hasn’t greatly changed things for point-source CO2 capture, but it did really affect direct air capture (DAC), or as they call it now “carbon dioxide removal.” As a result of this legislation, there’s significant funding potential for carbon removal, which didn’t exist a few years ago, it was very minimal before. We’ll probably see the effect more in the coming year as there will be more and more funding rounds from the DOE. Now the government just has to figure out how to spend the money. For point-source capture, there’s still a considerable amount of funding available for CO2 capture from power plants—both coal-fired and natural gas-fired. The government is also interested in industrial CO2 capture—for example, from cement or steel plants—as part of point-source capture. There are additional funding opportunities being announced for industrial CO2 capture more often now. Again though, the biggest change compared to three or four years ago is the focus on carbon dioxide removal—there’s quite a bit of research going in that direction both in academia and in industry.
Can you give an overview of the different carbon capture technologies being funded by the DOE and how commercially ready they are today?
Basically, for all carbon capture applications, we divide the technologies into three categories—solvents, membranes, and sorbents. They all are used in different types of CO2 capture applications. I’ll give the pros and cons for each category.
Solvent technologies are the most conventional, the ones that have been used for decades. So far, this class of technology has received the most funding from the government. Amine absorbers have been used for years and have good CO2 capture capacity. The material cost is also not so high. However, amine solvent systems require a lot of space and have quite a problem with heat and mass transfer, resulting in poor reaction kinetics and high energy costs. For a typical power plant using amine solvent-based carbon capture, it might require 1/3 of the power output of the plant just to regenerate the solvent and operate the technology. In addition, there are further issues with the viscosity and corrosivity of amine solvents. These issues are why people continue investigating them.
There are startups that are developing better solvents, both for point-source capture and for direct air capture. For direct air capture, maybe the largest CO2 capture capacity was offered by Carbon Engineering. They offer an alternative to the amine-based solvents and use a potassium hydroxide solvent, along with a novel contactor to get better kinetics and faster CO2 absorption and desorption cycles. Other startups are also trying to blend the solvents to get better capacities. Noya, for example, is adding chemicals to water to capture CO2 leveraging the cooling systems of factories and power plants. There’re quite a few people working on other alternatives to amine solvents, like PDMS- and ethylene glycol-based solvents, for capturing CO2 from streams with a high concentration of CO2, like in hydrogen production, for instance. So, there’s quite a bit going on with solvents. Noya and Carbon Engineering are targeting direct air capture, but there are still point-source capture and other types of capture being considered for solvents. Solvents are still the default technology, especially when you target large amounts of CO2 capture.
With membranes, you don’t have desorption or regeneration, that’s the biggest advantage over solvents and sorbents. It’s just a one-part system. Membranes are very good at capturing or separating CO2 for high-concentrations streams. The higher the CO2 concentration in the emission stream, the more beneficial the membrane becomes. Another advantage of membranes is that, unlike solvents, they can be processed into sheets or hollow fibers. Separating different types of gasses—nitrogen, oxygen, etc.—is a multi-billion-dollar industry, so there’s know-how coming from certain types of gas separation that is now being channeled into carbon capture. The problem with most membranes is the low capture capacity when it comes to low CO2 concentrations. Materials-wise, when you have a selective membrane, there’s a flux and permeability problem. When you have very permeable membrane, you have a selectivity problem. The target for everyone in the world is to overcome this tradeoff and get both a highly selective and a highly permeable membrane. Another problem with membranes is that as you make them thinner and thinner, you lose the selectivity. There are also other materials problems, such as aging.
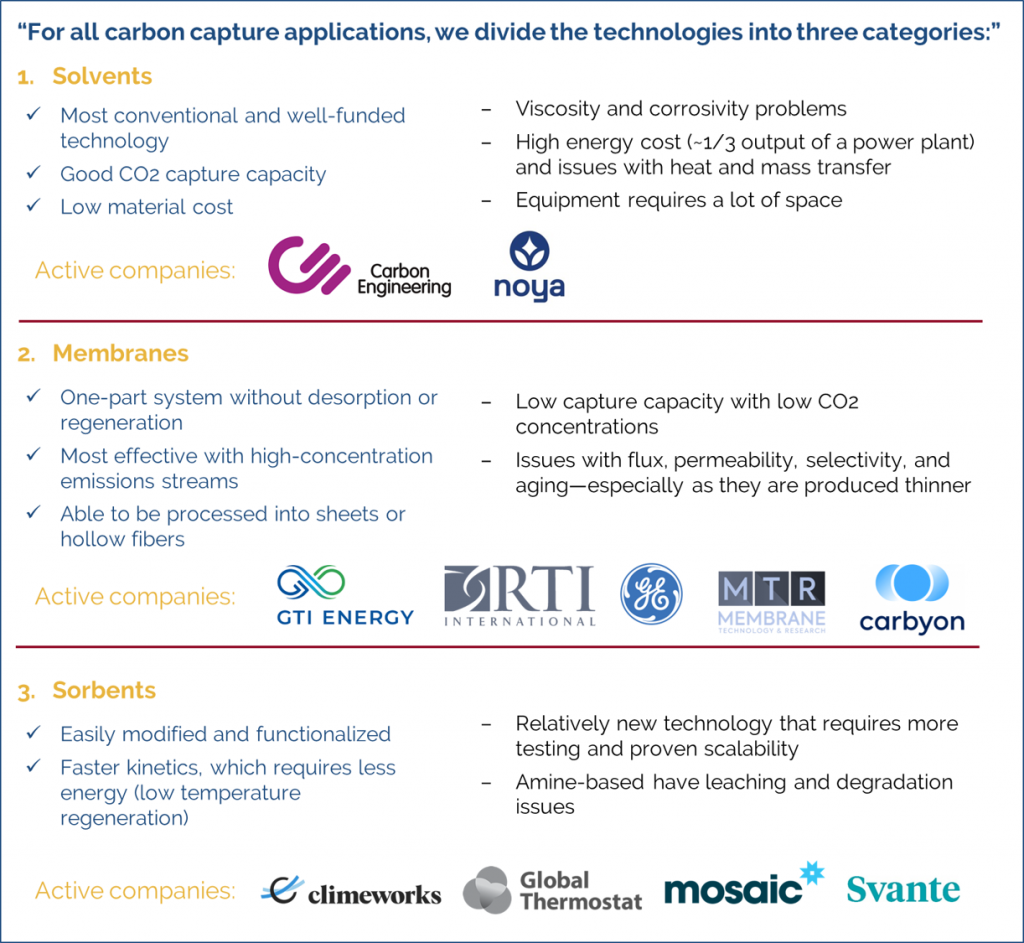
Companies developing membranes for CO2 capture include GTI Energy, RTI International, GE, and MTR. The front runner, in terms of performance, is the membrane called Polaris from MTR, which is polyethylene-oxide-based. GTI is working with Air Liquide on a polyether ether ketone (PEEK) membrane system. Overall, polymer-based membranes like these have the lead for membrane-based CO2 capture. There are some other types of membranes that are ceramic- and MOF-based, but they’re still being investigated for CO2 capture. For direct air capture, there are some startups, like Carbyon, that are seeding sorbents inside membranes. We call these mixed matrix membranes. The membrane in this case facilitates contact between CO2 and the sorbent. It also acts as a barrier, only allowing certain gases to the sorbent and preventing humidity-stable membranes from degrading.
The last big category is sorbents. Sorbents didn’t have a big portion of funding until a couple of years ago because the conventional way of capturing CO2 was with solvents. But when it comes to direct air capture specifically, most of the research is on sorbents. Sorbents are advantaged because they can be easily modified and functionalized. Their kinetics—mass and heat transfer—are also faster than solvents, so they require less energy. Sorbents are still relatively new technologies for CO2 capture though; more testing is needed, and scalability needs to be proven. Most sorbents for carbon capture are based on amines, which have problems in application. There’s amine leaching and oxidative degradation, both of which must be solved.
Many DAC companies are using amine-based solid sorbent systems. Climeworks is using amine-based inorganic sorbents in their system. Global Thermostat is another example; they’re also using amine-modified silica, like Climeworks, the idea is to use a high surface area material functionalized with amines to contact and capture CO2. Other companies, like Mosaic Materials, are working with materials called metal organic frameworks (MOFs); Mosaic Materials uses a magnesium-based MOF and then adds amines to boost the CO2 uptake capacity. When it comes to MOFs, the surface area is great, sometimes up to 10x better than classical zeolites, but the problem is scalability and cost. MOFs also degrade in acidic and humid environments, so stability is a big problem to be solved. Svante is another company developing MOFs. They developed a MOF called CALF-20 with researchers from the University of Calgary and are now producing it at the ton scale. CALF-20 offers very fast kinetics and good CO2 capacity, so it can be applied for direct air capture.
There are other types of sorbents, which we haven’t talked much about yet, that are metal oxide- or alkali-based. The basic idea is to use metal oxides, like calcium, potassium, or magnesium, to capture CO2. Heirloom, for example, is capturing CO2 with calcium carbonate rocks; they grind the rock and then spread it around to capture CO2, then calcinate it to activate the rock again. The problem with this technology is that you have to activate the material at very high temperatures—800 or 900 Celsius. Another startup doing promising work with solid alkali metal sorbents for direct air capture is Sustaera.

How would you say the DOE is looking at each of these three major buckets of technologies? Are they evenly paying attention to them?
It depends on the application. When it comes to coal- and natural gas-fired power plants, solvents research is dominant. When you move to industrial-based CO2 capture, membranes, solvents, and sorbents are all well-funded. For example, in natural gas processing, membranes are well funded, but so are sorbents and solvents. When it comes to direct air capture, most of the funding goes to sorbents, according to the funding award announcements. Solvents are also being considered for direct air capture while membrane research is very minimal. You may see studies talking about membrane-based direct air capture, but usually there’s sorbents inside the membrane. I’d still categorize these studies as sorbent-based direct air capture. To summarize, for power plant-based CO2 capture, I’d say the most funding has been awarded to solvents, for direct air capture it’s to sorbents, and membranes are sort of in the middle.
What is it about direct air capture that makes solid sorbents a better choice compared to a solvent or membrane? Why are most DAC startups looking at solid sorbents instead of the incumbent solvent technology today?
Based on the studies out there, the biggest reason is the kinetics. When it comes to direct air capture, you need to cycle your system a lot. Sorbents allow for that due to better mass and heat transfer. When it comes to solvent systems, there’s a large amount of liquid that you need to heat up and cool down, so a lot of energy is needed. For sorbents, you also have more room to develop the material, functionalize it how you want it. There are some promising solvents for DAC, but the literature shows that having low energy penalty is harder with solvents. That’s why Carbon Engineering is developing a special contactor to tackle that.
For point-source capture, do you think an emerging technology might displace the incumbent solvent technology?
When it comes to point-source capture, all technologies have a chance. It depends on the target CO2 capture capacity. We talked about capture capacity but at the end of the day, you need to talk about CO2 capture rate—how fast you’re capturing the CO2. So, when it comes to that, sorbents also have a chance. Membranes always have a chance for point-source capture because of hybrid systems where you combine membranes with solvents. RTI or GTI are doing this, removing some part of a gas stream with a membrane, and then sending it to a solvent. They can be integrated very well.
As a research scientist that has looked at many different types of carbon capture technologies, what emerging technology—either for point-source capture or direct air capture—do you find exciting?
CALF-20 MOF from Svante has shown promising performance according to recent publications. They talk about the scale up of MOFs, which has never been talked about before. Svante is one of the first to make it happen. They’re partnering with BASF, I think, to scale up, so they have an industry partner. I’m very curious to see what else is coming from Svante’s CALF-20 technology. For Climeworks and Global Thermostat, their amine-based system has a big advantage with scalability. You can make lots of material at a cheaper price. However, they need to improve the kinetics. I think if they can use a lower amount of amine in an alumina or silica sorbent with more effective amine concentration, it could be very interesting. For solvents, I think blended options have a better chance. Blending solvents together to lower viscosity, that’s interesting. For membranes, MTR’s technology is the frontrunner in terms of capacity, and it is polymeric, which means they can scale up. I think they have a big chance at succeeding, especially for high CO2 concentration capture.
[If I were a chemical or materials science company looking to get involved in carbon capture,] I’d start with the generic materials because in materials research it’s a long process going from discovery to commercialization. There are already very good materials out there.
If you were a company looking to get involved in carbon capture—say a chemical or materials science company—where would you place bets? What sorts of technology would you get involved with or specific chemistries?
I’d start with the generic materials because in materials research it’s a long process going from discovery to commercialization. There are already very good materials out there—amine-based, alumina, silica, CALF-20 MOFs, some metal oxide systems—I would choose 1-2 materials from each class and try to add some functionalities depending on the capture application. This is where I would start as a company trying to develop a new capture material. When you choose a generic material, there’s a problem in that the patents and rights could be owned by someone. Many companies are getting around this by using open-source materials, like zeolites, and making novel system components like contactor arrays. Porous carbon is another good starting material because everyone can make their own by changing reaction conditions. If you’re trying to make a proprietary material that could be a good starting point.
Last question, when you look at the emerging technologies today, what breakthroughs do you think the industry still needs? What are the key challenges if you were to generalize across the different technological classes?
The critical criteria for new technologies are CO2 capture capacity, CO2 selectivity, regeneration temperature, and cyclability. Together, these give you cost efficiency. A comprehensive approach should be followed by a company; new technologies should pay attention to all the criteria. People have reported record amounts of CO2 capture capacity, but if you can’t cycle it in a less expensive way, that’s a problem. High CO2 capture capacity is one thing to start with material research, but you need to maintain that capacity in a cost-efficient way. Also, how fast you capture and how fast you release is important—it’s not just about how much you can capture, the rate matters. New materials also have to be scalable. If you find an exotic material and achieve the criteria but can’t scale up, then it doesn’t matter. These are the things that companies should consider rather than focusing on just one parameter of a material.
Thanks for taking the time to talk with us today.
