A sad truth is that rare diseases aren’t really all that rare. This classification refers to any disease affecting less than 200,000 individuals – but with over 7,000 diseases qualifying for this definition, there are currently 20-35 million Americans living with some form of a rare disease. While many are genetic and appear at birth, some don’t show until later in life (e.g., Huntington’s disease usually displays in someone’s mid-50s). A lot of these diseases do not yet have treatment, but the advent of cheap genomic sequencing is helping to address that problem by finding the genetic causes of these diseases where previously they were unknown. These new discoveries, combined with cell and gene therapies for rare diseases and cancer, could potentially be the next great breakthrough in medicine.
One of most promising areas of innovation in cell and gene therapies are viral vectors. A viral vector is a delivery mechanism for treatment that uses an engineered version of a naturally occurring virus to carry the therapeutics where they’re needed. To make a viral vector, DNA encoding for the desired therapy is introduced into a virus-producing cell line. These cell lines are cultured in high-volume bioreactors over several days, secreting the therapy-containing virus. The virus is then isolated and purified to create the viral vector end product.
Viral vectors produced from this process can be administered in one of two ways:
- In Vivo Delivery: This method involves injecting viral vectors directly into patients. It is mostly used for patients whose rare disease causes mutations in an essential protein, thereby producing a variety of symptoms (e.g., Duchenne muscular dystrophy [DMD], a disorder caused by a genetic mutation that limits production of a protein called dystrophin and results in progressive muscle cell breakdown). The viral vectors travel to the mutated cell of interest and deliver a working copy of the compromised protein. This process can be essentially curative for variety of different diseases rather than just treating the symptoms (as often happens in modern medicine).
- Ex Vivo Delivery: In this approach, immune or stem cells are taken from a patient, cultured in a lab, and given the viral vector to introduce the desired therapy into the cells. The cells containing the therapy are then reintroduced into the patient’s body. This process has been increasingly used in cancer treatments (e.g., Kymriah, Yescarta, Tecartus), delivering engineered receptors to T cells to target cancers with incredible efficacy.
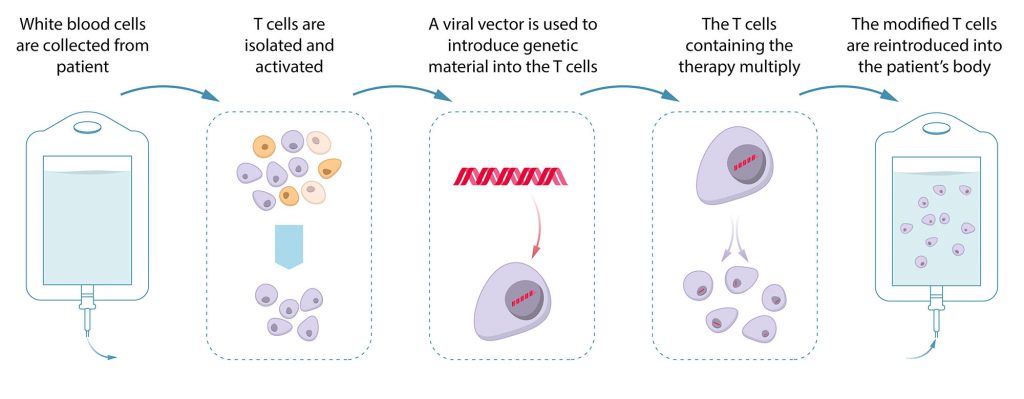
Process overview of ex vivo delivery of viral vectors in cell and gene therapy
While both mechanisms show tremendous therapeutic potential, their development has been somewhat inhibited by the economics of treating rare disease. Major pharmaceutical companies do not have a financial incentive to manufacture treatment for diseases affecting such small patient populations, especially when many of the most promising treatments are curative (i.e., one-time use). As a result, most innovation is emerging from small, less well-resourced startups, and these companies are asking top dollar for their products (the average cost of currently available gene therapies is $1.4M).
To make matters worse, insurers often resist footing the bill when faced with such an extreme price tag. They may require companies to provide special rebates or lottery programs for doses, which only disincentivizes development further. Fortunately, increased automation and technology maturity should bring treatments down the cost curve, making them more available to the patients who need them.
The cell and gene therapy market is set to explode in the coming years. Up to the early 2010s, most trials were unsuccessful, but researchers have been steadily gaining traction since then (likely due to a shift in which viral platforms are being used, from more generally infectious lentiviruses to highly targeted AAV and Adenoviruses). While most of these experimental treatments are in pre-clinical trials and not yet being tested in humans, over 3,000 new therapies are currently in development as of 2022 and three curative offerings (e.g., muscular atrophy) have been approved and are on the global market.
This potentially massive market is poised to be the next great modality in medicine and give patients without options a chance to receive the treatment they need.
Find out how Newry can help your organization find high-value options for growth. Expand your pipeline before it runs dry.
